Call: H2020-MSCA-ITN-2015 Topic: MSCA-ITN-2015-ETN Project / GA number: 675074 Project full title: Protein-excipient Interactio
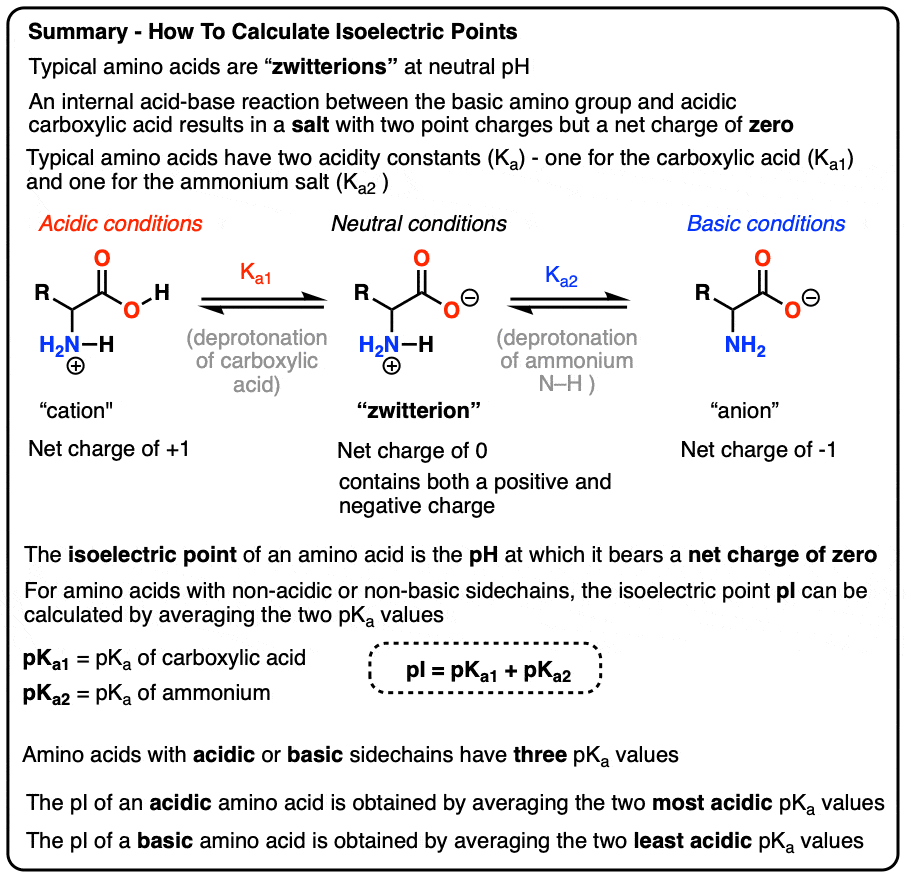
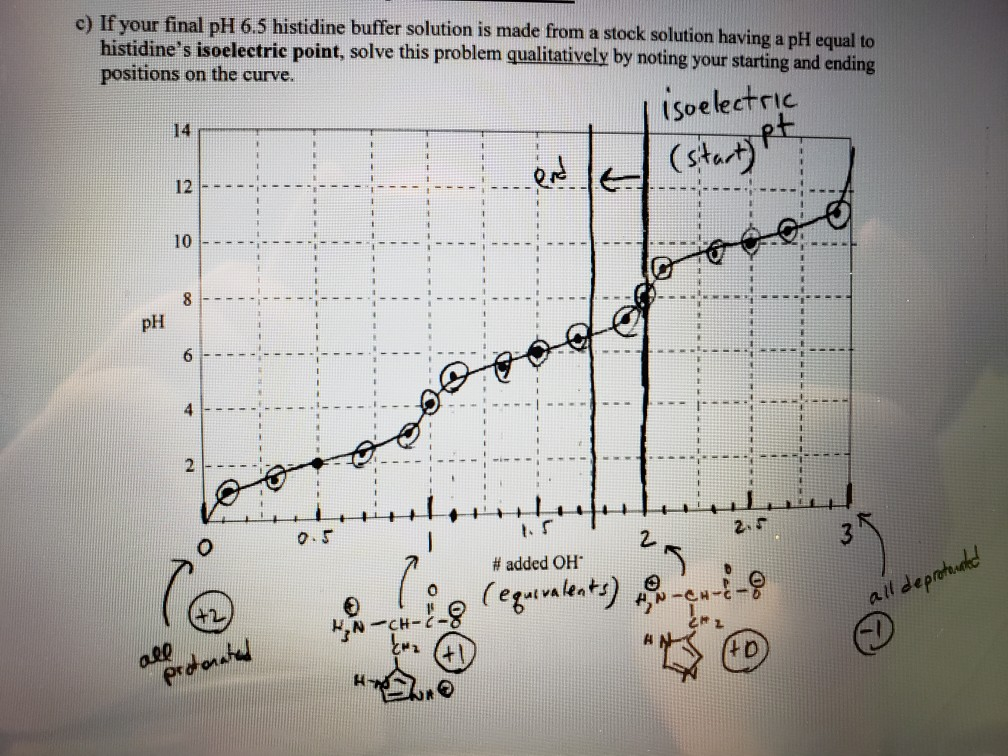
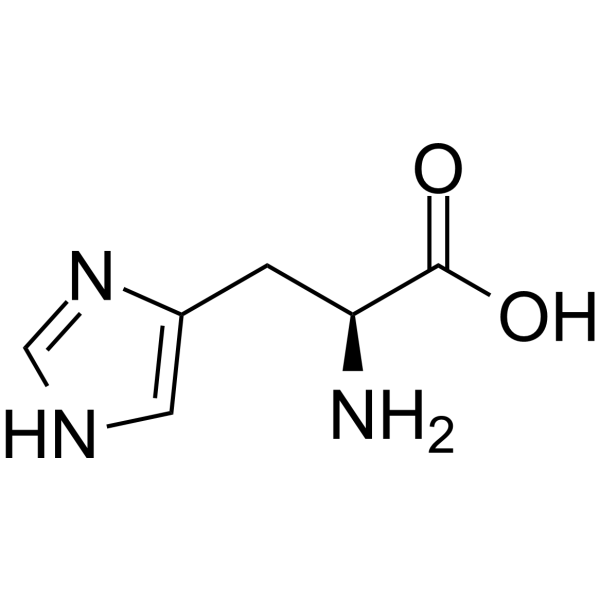
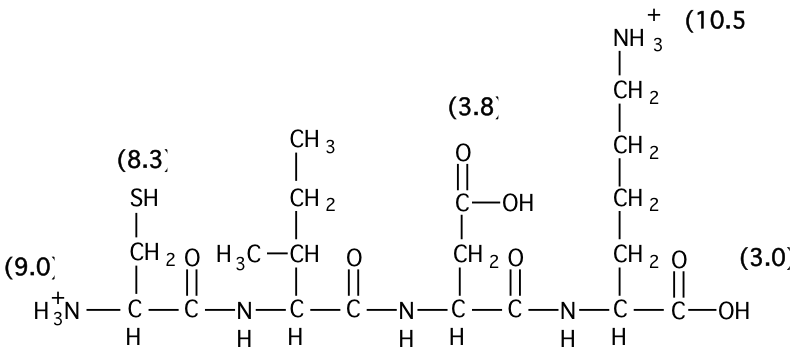
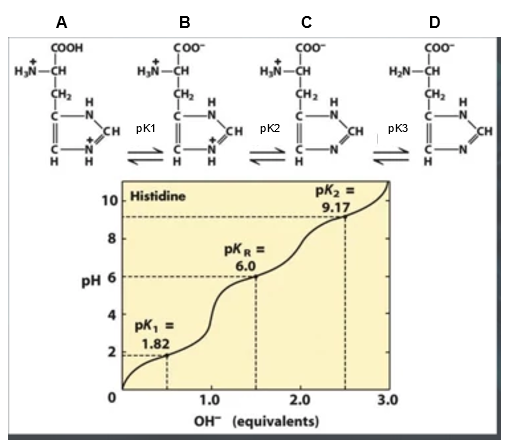
SOLVED: 20. Calculation of the pH of a Solution of a Polyprotic Acid Histidine has ionizable groups with pKa values of 1.8, 6.0, and 9.2, as shown below (His imidazole group). A

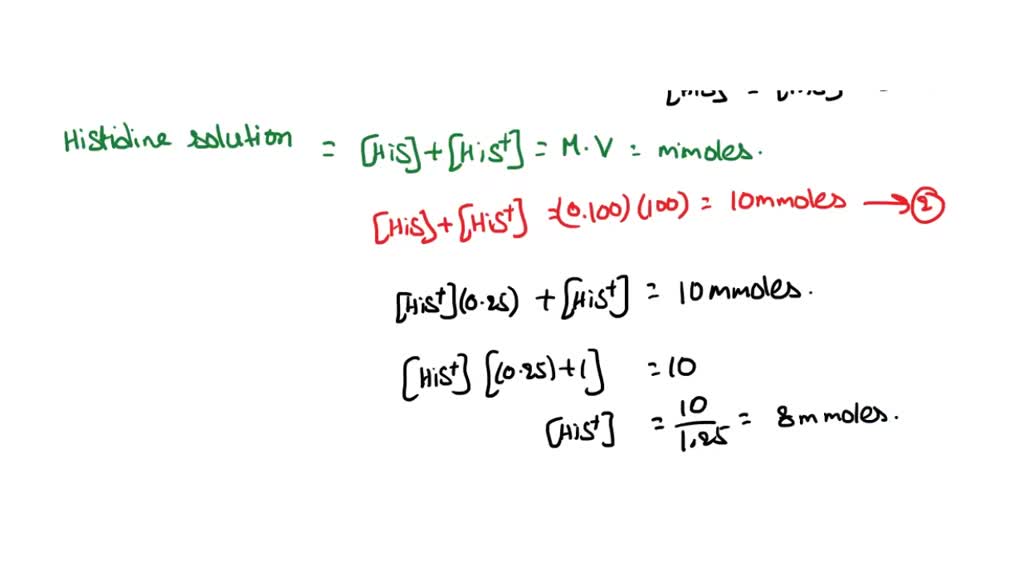
physical chemistry - Calculating the ionic strength of a histidine solution - Chemistry Stack Exchange
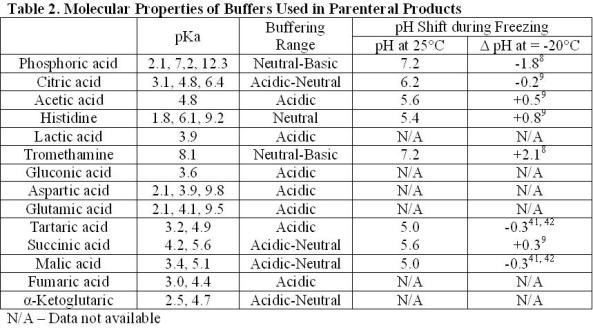
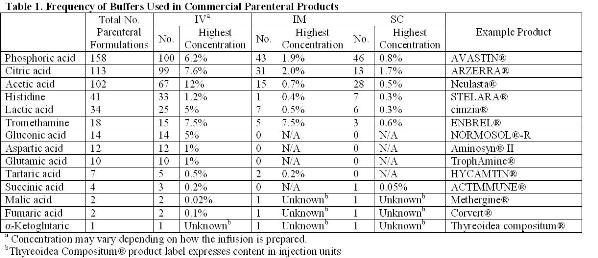
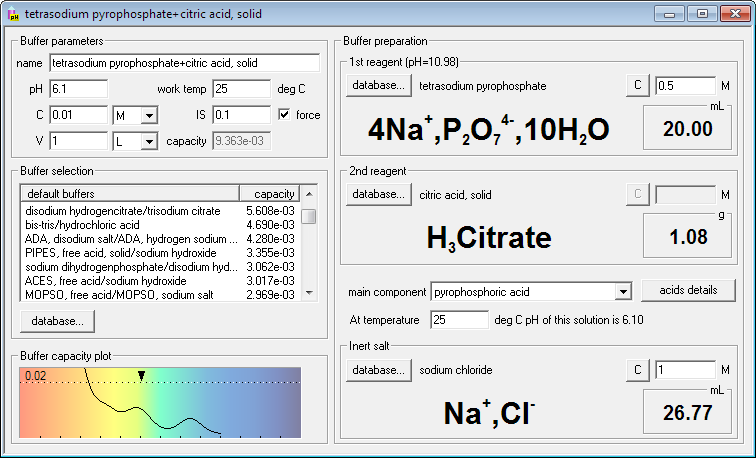
Breaking old habits: Moving away from commonly used buffers in pharmaceuticals - European Pharmaceutical Review
How is histidine the only amino acid whith a side chain that ionizes within a physiological pH? - Quora
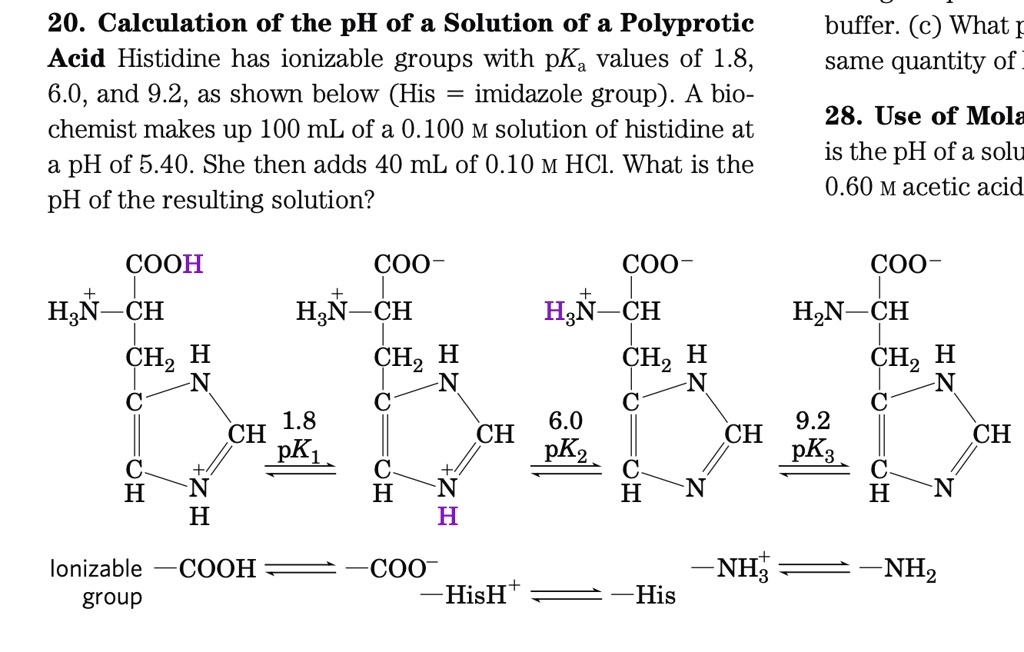
SOLVED: 20. Calculation of the pH of a Solution of a Polyprotic Acid Histidine has ionizable groups with pKa values of 1.8, 6.0, and 9.2, as shown below (His imidazole group). A

Change in pH as a function of temperature for histidine acetate buffer... | Download Scientific Diagram

Effect of Buffer on Protein Stability in Aqueous Solutions: A Simple Protein Aggregation Model | The Journal of Physical Chemistry B